Knock, Knock, FDA Calling
In the United States, the Food and Drug Administration (FDA) is responsible for protecting and promoting public health with ultimate oversight of food and drugs, and of particular interest for this article, the nutritional supplement industry. Their regulatory control covers two main areas: product safety and accurate product labeling. This article summarizes some of what goes on behind the scenes to keep consumer products safer.
The Dietary Supplement Health and Education Act of 1994 mandated that the FDA regulate dietary supplements as foods, rather than as drugs. This distinction means that dietary supplements are not subject to approval by demonstrating safety and efficacy as is the case with drug approvals. The FDA can take action against dietary supplements only after they are proven to be unsafe. Despite this "lower" standard of control as compared with drugs, manufacturers of nutritional supplements are held to a high standard and are required to follow current Good Manufacturing Practices (cGMP). The FDA oversees facility approvals.
A manufacturer of a drug is also permitted to make health claims about the drug, such as "cures bacterial infection" whereas a nutritional supplement can be described through a "structure or function" claim, such as supports a strong immune system". A manufacturer may not claim a nutritional supplement to treat, diagnose, cure, or prevent disease, as reflected in the ubiquitous disclaimer on the label.
The FDA exerts control over products on the market by targeting the companies that produce them through inspections of manufacturing facilities, analysis of business practices and label claims. Any divergence from established law will result in an FDA letter that can be accompanied by a variety of penalties or stipulations for change. An FDA letter would be received by a manufacturing facility that fails to meet any significant current Good Manufacturing Practice (cGMP). Such harshly-worded letters include an extensive list of the infractions and a deadline by which the company must provide satisfactory evidence of resolution or risk being shut down by the FDA. A serious infraction, such as drug contamination that causes illness, can result in the FDA shutting down a facility immediately or to request a hold on specific drug lots.
Another FDA guideline involves product labeling. Under Title 21, Code of Federal Regulations, Part 101 (21 CFR Part 101), misbranded products are subject to control as well. As dietary supplements are limited in their label claims as well as require total accuracy reflecting the contents of the product, the FDA can object to specific language on a label. Such was the case in 2011 with a popular product "Muscle Milk". The FDA issued a warning letter to the product's manufacturer, Cytosport, indicating an objection with the label language perhaps containing a confusing message to consumers that the product contained milk, when in fact it does not contain milk but does contain milk-derived ingredients. The FDA requested updated product labeling and supporting marketing materials to further clarify the product contents. The FDA also identified some other label claims with which it disagreed. The investigation is ongoing, but it's worth noting that is does not relate to the safety of the product but rather to the labeling.
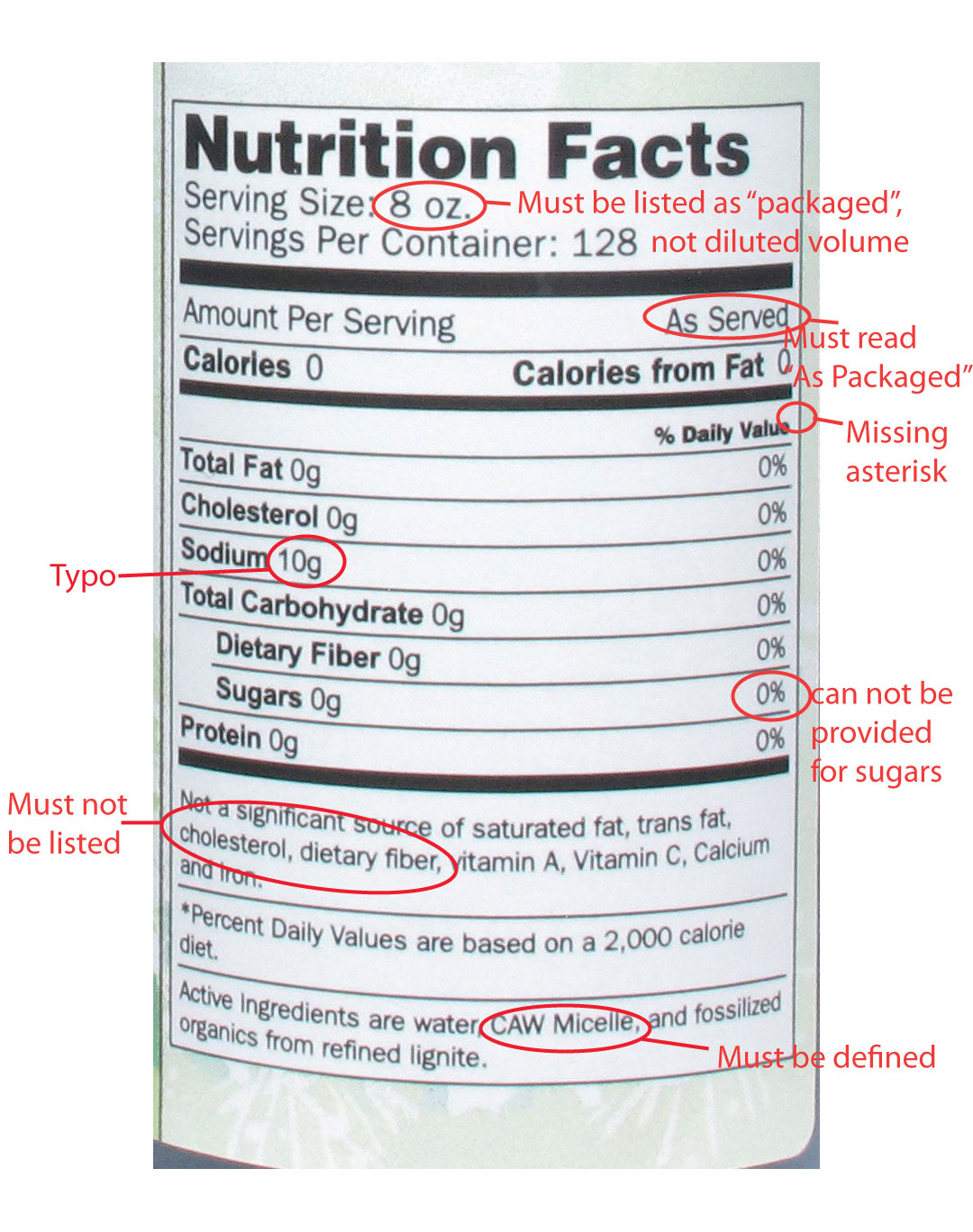
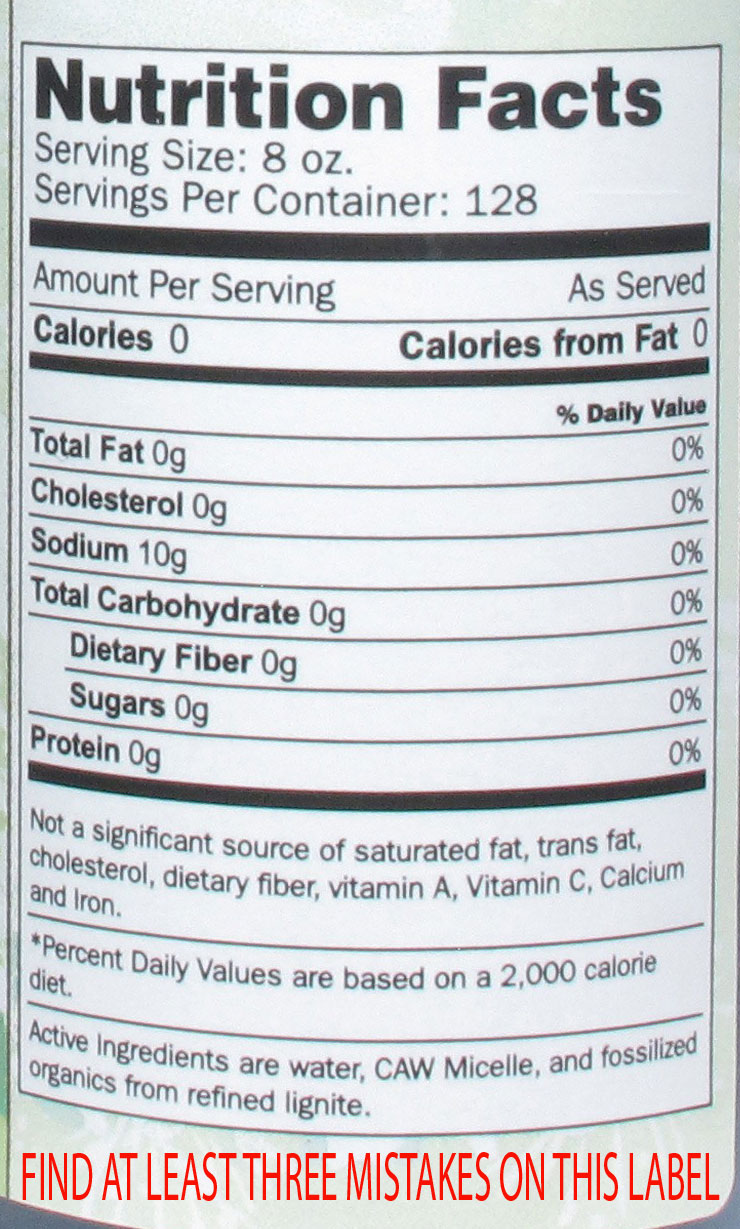
Take for example another product containing an antioxidant from another company. The bottle label says "very powerful antioxidant". In a recent action letter, the FDA wrote "The claim "very powerful antioxidant" as used in your labeling is an unauthorized nutrient content claim. The term "very powerful" characterizes the level of antioxidant nutrients in your product, and therefore, this claim is a nutrient content claim." The letter goes on to state "Your products declare Sodium as 10g, which, in accordance with 21 CFR 101.9(c)(4), must be expressed in milligrams. Further, we note that 10 g of sodium is not consistent with a Daily Value of 0%." It is these sort of claims and label errors that are cause for alarm, and thankfully, the focus of FDA inspections and oversight. As athletes, we are can be faced with a barrage of nutritional supplements with all sorts of wild claims. If something sounds too good to be true, it probably is. Read labels of products and make an informed decision. If you notice errors and inconsistencies in the label, or claims that make you think "this sounds like a drug" then it's probably best to give that product a pass and move onto something else.
The FDA also inspects manufacturing facilities, and can issue "483" observation or warning letters to facilities that are significantly out of compliance. Most FDA violations involve one of the following:
1. Not having procedures in a regulated area that conform to FDA regulations;
2. Having procedures that conform to FDA regulations, but not following them; or
3. Having procedures that conform to FDA regulations and following them, but not having adequate documentation to show that you’re following them.
Recent and past FDA warning letters make for some interesting reading, and are located here: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/
A warning letter clearly indicates the systems or products of concern, and requests a response within a certain period of time. Failure to respond, or to respond inadequately, can result in the FDA imposing penalties, fines or a facility closure. Once the FDA has completed an evaluation of corrective actions via follow-up inspection, it may issue a Warning Letter "close-out letter".
It should be somewhat reassuring that government organizations continue to maintain awareness for public safety through a regulated food supply. While there are still strides to be made towards improving the program and regulatory resources, as well as increased inspections and disciplinary actions, our food supply is still overwhelmingly safe.
References:
FDA Muscle Milk label warning letter:
http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2011/ucm261684.htm
FDA Powerful antioxidant warning letter:
http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm290046.htm
Lots of great information and references via the FDA website: http://www.fda.gov
Jonathan Toker is the Slowtwitch.com science editor and an elite-level runner-triathlete who hails from Canada and lives in Southern California. He received a Ph.D. in organic chemistry from The Scripps Research Institute in 2001. Jonathan invented the SaltStick products in 2002. www.SaltStick.com


